Description
Abbreviation: TPEs
Applications
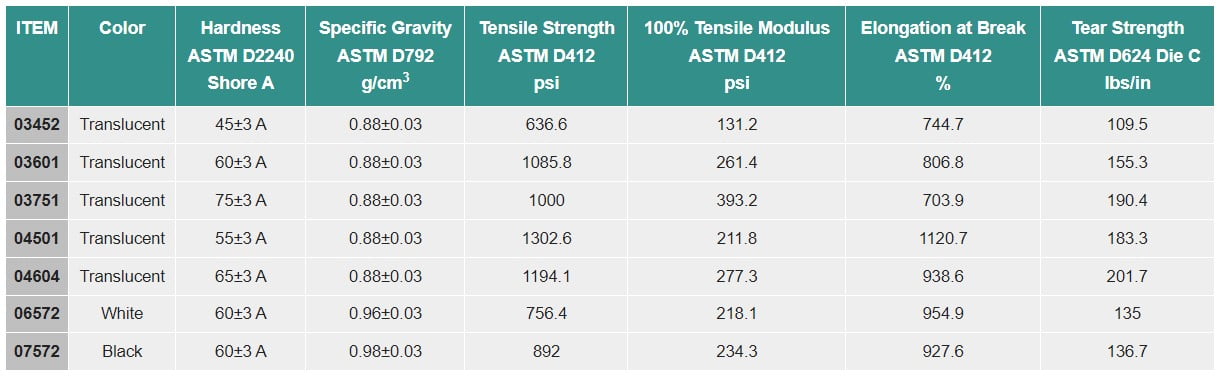
PHOENIX™ Translucent Thermoplastic Elastomers (TPEs) 04 Series
medical extrusion, medical tubing, catheter, uterine balloon tamponades
PHOENIX™ White Thermoplastic Elastomers (TPEs) 06 Series
single use, disposable syringe gasket / syringe plunger seals
soft touch parts/components of medical devices
glass vial caps
PHOENIX™ Black Thermoplastic Elastomers (TPEs) 07 Series
disposable syringe gasket / syringe plunger seals
soft touch parts/components of medical devices
glass vial caps
Safety/Quality Approvals:
ISO 10993-5 Cytotoxicity Test
ISO 10993-10 Guinea Pig Skin Sensitization Test
ISO 10993-23 Rabbit Skin Irritation Test
FDA CFR 21 177.2600, RoHS, EN71, REACH SVHC, PAHS & phthalate, Formaldehyde, Dimethyl Fumarate, Nonylphenol
Details: PHOENIX™ medical grade product lines are ideal for a range of medical device applications in the medical and pharmaceutical industries because they comply with international biocompatibility regulations. For a variety of applications, PHOENIX™ Thermoplastic Elastomers (TPEs) are now offered in transparent, translucent, white, and black colors.